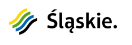
Rys. 1 Schemat spektrometru mas z analizatorem https://pl.wikipedia.org/wiki/Spektrometria_mas
The history of mass spectrometry began with the research of many scientists into the conduction of electric current through gases and the associated glow. In 1886 Eugen Goldstein discovered anode radiation, and in 1898 Wilhelm Wien noticed that radiation refracted in a strong magnetic field splits into separate beams. He discovered that when the gas was discharged at low pressure, there was a glow behind the holes in the anode. Such research, conducted by Joseph John Thomson of Cambridge University, led to the discovery of the electron in 1897. Thomson was awarded the Nobel Prize in Physics in 1906. In 1899, Wilhelm Wien introduced a device (Vienna Separator) in which ions moving in a perpendicular electric and magnetic field, emitting ions at a certain velocity, were deflected in the magnetic field itself, separating according to the ratio of charge to mass. Wien stated that the charge-to-mass ratio depends on the type of gas in the pipe. In 1911, Thomson improved the Vienna system by reducing pressure and creating a mass spectrograph. Using mass spectrography, he discovered neon isotopes in 1913.
In the early 20th century, Thomson discovered that cathode rays (electron beams) could be deflected by an electrostatic field. The device in which this phenomenon was observed is a precursor of the mass spectrometer. Thomson did not limit himself to observing the bending of the electron beam, but began to study the bending of beams of different ions. These studies led to the creation of the first mass spectrometer in 1899-1911, which Thomson called a parabolic spectrograph. In this device, the ions were exposed to parallel electric and magnetic fields, causing their trajectories to deviate. Ions of different energies had different deviations parallel to the field line, and ions of different momentum perpendicular to the field line. The detector of the Thomson spectrometer was a photographic film or a fluorescent screen. Ions with the same m/z ratio formed an image on the screen in the form of parabolas with a certain parameter; the appearance of several parabolas with different parameters was evidence of the existence of isotopes.
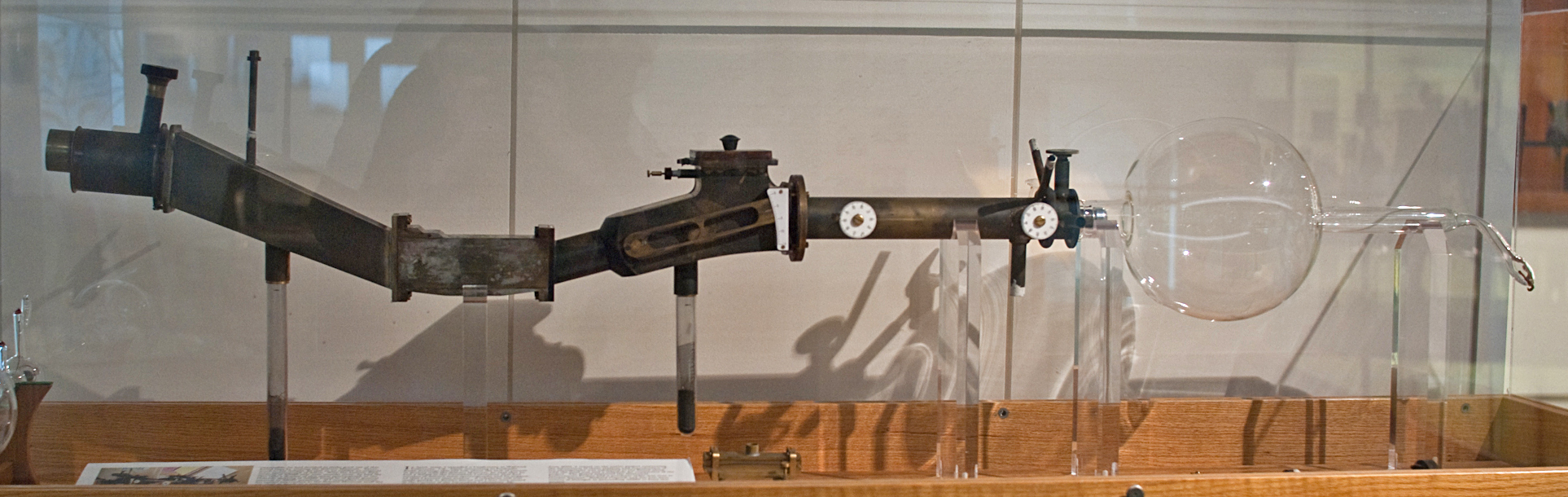
Rys. 2 Replika spektrometru Thomsona
https://pl.wikipedia.org/wiki/Spektrometria_mas

Rys. 3 Wysokorozdzielczy spektrometr mas https://pl.wikipedia.org/wiki/Spektrometria_mas
After World War I, Thomson's colleague, Francis William Aston, built a mass spectrometer with a much higher resolution. This spectrometer made it possible to observe isotopes. Aston received the Nobel Prize in Chemistry in 1922 for building a mass spectrometer and discovering many non-radioactive isotopes. At the same time, Arthur Jeffrey Dempster, like Aston, was working on a magnetic analyzer. Dempster has also developed a method of ionizing the substance using an electron current (the source of NO ions), which is still in use.
Thomson, Aston and Dempster laid the theoretical foundations for the development of mass spectrometry. Today, more than a century after Thomson’s first experiments, mass spectrometers are an indispensable tool in the work of physicists, chemists, biologists and doctors conducting scientific research on Earth and in space. Mass spectrometers are increasingly used in industry, military, law enforcement and other institutions. Molecular biology is one of the fields that make extensive use of mass spectrometry. The analysis of large-molecule biopolymers, such as proteins and nucleic acids, would not be possible without mild ionization methods – electrospray (ESI) and matrix-assisted laser desorption (MALDI). In 2002, the Swedish Academy of Sciences awarded the Nobel Prize in Chemistry to John Fenn and Koichi Tanaka for their use of light ionization methods in the study of large molecular biopolymers.

Rys. 4 Spektrometr MALDI-TOP ze zwierciadłem jonowym https://pl.wikipedia.org/wiki/Spektrometria_mas