Meitner is an extremely radioactive synthetic element with the atomic number 109.
In the periodic table, meitner is a transactin d-block element. It is a member of the seventh period and is placed in the group of 9 elements, although no chemical experiments have yet been conducted to confirm that it behaves like the heavier homologue of iridium in group 9 as the seventh member of the 6d series of transition metals. Meitner is calculated to have similar properties to its lighter homologues, cobalt, rhodium and iridium.
About the story of discovering the words of the clique. . .
Meitner was first synthesized on 29 August 1982 by a German research team led by Peter Armbruster and Gottfried Münzenberg at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung) in Darmstadt. The team bombarded the shield of bismuth-209 with accelerated iron-58 nuclei and detected a single atom of the isotope meitner-266:
This work was confirmed three years later at the Joint Institute for Nuclear Research in Dubna (then in the Soviet Union).
Why the name "meitner"?
Using Mendeleyev's nomenclature for unnamed and undiscovered elements, meitner should be known as eka-iridium. In 1979, during the transfer wars, the IUPAC issued recommendations that the element should be called unnilennium (with the corresponding symbol Une), a systematic name for the element as a surrogate, until the element was discovered (and then confirmed) and a decision was made on a permanent name. Although these recommendations were widely used in the chemical community at all levels, from chemical classes to advanced textbooks, they were largely ignored by scientists in the field, who either called it “element 109”, with the symbol E109 or even simply 109, or used the proposed name “meitnerium”.
 The name meitner has been discussed in controversy over the naming of elements 104 to 109, but meitner was the only proposal and was therefore never questioned.The name meitner (Mt) was proposed by the GSI team in September 1992 in honor of Austrian physicist Lise Meitner, co-discoverer of protactin and one of the discoverers of nuclear fission. The name was recommended by IUPAC in 1994 and was officially adopted in 1997.
The name meitner has been discussed in controversy over the naming of elements 104 to 109, but meitner was the only proposal and was therefore never questioned.The name meitner (Mt) was proposed by the GSI team in September 1992 in honor of Austrian physicist Lise Meitner, co-discoverer of protactin and one of the discoverers of nuclear fission. The name was recommended by IUPAC in 1994 and was officially adopted in 1997.
Does such an element have its isotopes?
Meitner has no stable or naturally occurring isotopes. Several radioactive isotopes were synthesized in the laboratory, either by melting two atoms or by observing the decay of heavier elements. Eight different isotopes of meitner have been reported with mass numbers 266, 268, 270 and 274-278, of which two, meitner-268 and meitner-270, have unconfirmed metastable states. The ninth isotope with a mass number of 282 is unconfirmed. Most of them decay primarily by alpha decay, although some undergo spontaneous cleavage
All isotopes of Mechner are extremely unstable and radioactive; in general, heavier isotopes are more stable than lighter ones. The most stable known isotope of meitner, 278-Mt, is also the heaviest known isotope; its half-life is 4.5 seconds. The unconfirmed isotope 282-Mt is even heavier and appears to have a longer half-life of 67 seconds. The isotopes 276-Mt and 274-Mt have half-lives of 0.62 and 0.64 seconds, respectively. The remaining five isotopes have half-lives ranging from 1 to 20 milliseconds.
The isotope 277-Mt, formed as a final decay product of 293-Ts for the first time in 2012, has been observed to spontaneously cleave with a half-life of 5 milliseconds. Preliminary analysis of the data considered the possibility that this fission could originate from 277-Hs, which also has a half-life of several milliseconds and could be filled after undetected electron capture somewhere in the decay chain. This possibility was later considered very unlikely based on the observed decay energies of 281-Ds and 281-Rg and the short half-life of 277-Mt, although some uncertainties remain. Regardless, the rapid cleavage of 277-Mt and 277-Hs strongly suggests the existence of a region of instability for superheavy nuclei of N=168-170. The existence of this region, characterized by a decrease in fission barrier height between the deformed shell closure at N = 162 and the spherical shell closure at N = 184, is consistent with theoretical models.
What properties can meitner have?
Apart from the nuclear properties, no properties of meitner or its compounds have been measured; this is due to its extremely limited and expensive production and the fact that meitner and its parent compounds decay very quickly. The properties of meitner metal remain unknown and only predictions are available.
Little attention has been paid recently to predicting the likely chemical properties of meitner. Meitner is expected to be a precious metal. The standard electrode potential for Mt3+/Mt is expected to be 0. 8 V. Based on the most stable oxidation rates of the lighter elements of group 9, the most stable oxidation rates of meitnerium are predicted to be the states +6, +3 and +1, with the state +3 being the most stable in aqueous solutions. For comparison, rhodium and iridium show a maximum degree of oxidation of +6, while the most stable states are +4 and +3 for iridium and +3 for rhodium. The degree of oxidation +9, represented only by iridium in [IrO4]+, may be possible for its meitner congener in fluoride-free (MtF9) and cation [MtO4]+, although [IrO4]+ is expected to be more stable than these meitner compounds. Meitner tetrahalides are also expected to have similar stability to iridium, thus enabling a stable +4 state. It is further expected that the maximum oxidation rates of the elements from bohr to darmsztadt may be stable in the gas phase, but not in the aqueous solution.
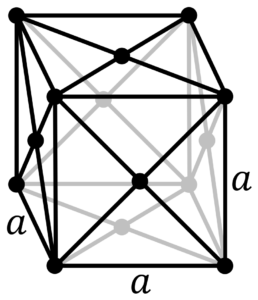 Meitner is expected to be a solid under normal conditions and adopt a cubic crystal structure, as is its lighter iridium congener. It should be a very heavy metal with a density of about 27-28 g/cm3, which would be one of the highest of the 118 known elements. It is also predicted that the meitner will be paramagnetic and that the covalent radius of the meitner will be 6 to 10 μm larger than the iridium radius, and its atomic radius is expected to be about 128 μm.
Meitner is expected to be a solid under normal conditions and adopt a cubic crystal structure, as is its lighter iridium congener. It should be a very heavy metal with a density of about 27-28 g/cm3, which would be one of the highest of the 118 known elements. It is also predicted that the meitner will be paramagnetic and that the covalent radius of the meitner will be 6 to 10 μm larger than the iridium radius, and its atomic radius is expected to be about 128 μm.