https://pl.wikipedia.org/wiki/Antoine_Lavoisier
Antoine Laurent de Lavoisier, chemist and physicist, born August 26, 1743 in Paris, independently of Johann Helmont and Mikhail Lomonosov, reformulated and disseminated the law of mass conservation, proved that oxygen is necessary for combustion and the production of acids (from which the old name "acidode" was derived), proved the theory of phlogiston and refuted the ancient theory of the elements as the basic components of matter and influenced the transformation chemical nomenclature.
He attended Mazarin College, where he studied chemistry, botany, astronomy and mathematics. In 1767, he worked as a geologist in Alsace and Lorraine. In 1768 he was elected a member of the French Academy of Sciences. In 1771 he married then 13-year-old Marie-Anne Pierrette Paulze, who translated for him from English and illustrated his books, later playing a key role in the management of both experiments and the standardization of scientific methods.
From 1775 he worked in the administration of the Royale des poudres, where his work improved gunpowder production and invented a new method of salt production.
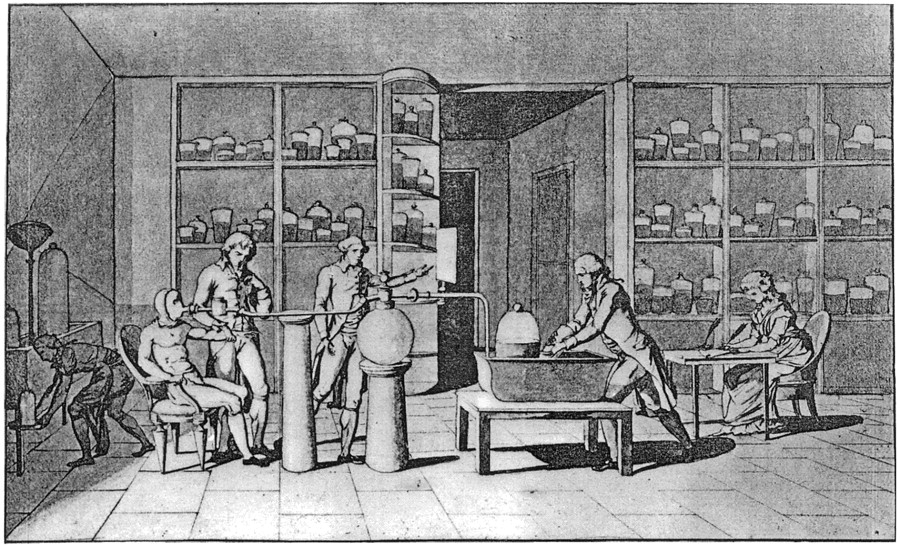
Lavoisier's most important experiments concerned the nature of ignition and combustion. They have shown that these processes involve combining the substance with oxygen. He also showed that oxygen plays a key role in animal and plant respiration and metal rusting. Lavoisier's explanations refuted the phlogistone theory, according to which a substance called phlogistone is released from materials during combustion. He also discovered that hydrogen combines with oxygen to form water. In Sur la burning en général and Decisionations Générales sur la Nature des Acides he showed that the ‘air’ involved in the combustion process is also a source of acidity. In 1779, he called the oxygen responsible for combustion “air” and the rest air nitrogen. In Reflexions sur le Phlogistique Lavoisier demonstrated the contradictions of phlogiston theory.

https://pl.wikipedia.org/wiki/Antoine_Lavoisier

https://en.wikipedia.org/wiki/Antoine_Lavoisier
Lavoisier's experiments were among the first to use a quantitative method. He showed that despite changes in the state of matter during the reaction, its quantity (mass) is the same before and after the chemical reaction. By burning phosphorus and sulfur, he noticed that the reaction product weighed more than its predecessors. He showed that excess weight is compensated by the loss of air weight. These experiences led to the formulation of the law of mass conservation. Lavoisier also studied the chemical composition of water, which he called oxygen and hydrogen. In collaboration with French scientist Claude-Louis Berthollet, Lavoisier developed a chemical nomenclature. A large part of the nomenclature is still in use, for example: sulphuric acid, sulphate.
His Traité Élémentaire de Chimie is considered the first modern textbook of chemistry; it contains Lavoisier's views on chemical theory, formulates the law of mass conservation, and denies the existence of phlogiston. Lavoisier also refines the concept of an element, defining it as a simple substance that cannot be broken down into components by any method of chemical analysis. He also formulated the theory of combining elements into chemical compounds. The user manual also lists the following elements: oxygen, hydrogen, nitrogen, phosphorus, mercury, zinc and sulfur. The list also included light and heat, which Lavoisier also considered substances.
Lavoisier's work is considered to be the definitive overthrow of the ancient Greek view of the five elements, which are supposed to be the basic building blocks of matter (water, fire, air, earth, ether).
https://en.wikipedia.org/wiki/Antoine_Lavoisier

https://en.wikipedia.org/wiki/Antoine_Lavoisier
Of great importance in Lavoisier's life was his interest in law, which gave rise to his interest in politics. At the age of 26, he started working as a tax collector at the private tax collection company Ferme générale, where he tried to make changes to the financial and tax system in France. Working for the government, he developed a system of measures to ensure uniformity of scales throughout France. As one of the 28 French tax collectors, he was considered a traitor by the revolutionaries and guillotined in Paris in 1794. At the request of a scientist, his execution was postponed for several days because of his work on a scientific experiment, the president declared a court.
At the time of his death, Antoine Lavoisier made a final attempt - he agreed with his assistant that he would try to blink for as long as possible, according to the assistant's story, he blinked for 15-20 seconds.
In 1788, he became a Fellow of the Royal Society of London, and his name was included in the list of 72 names of the Eiffel Tower.